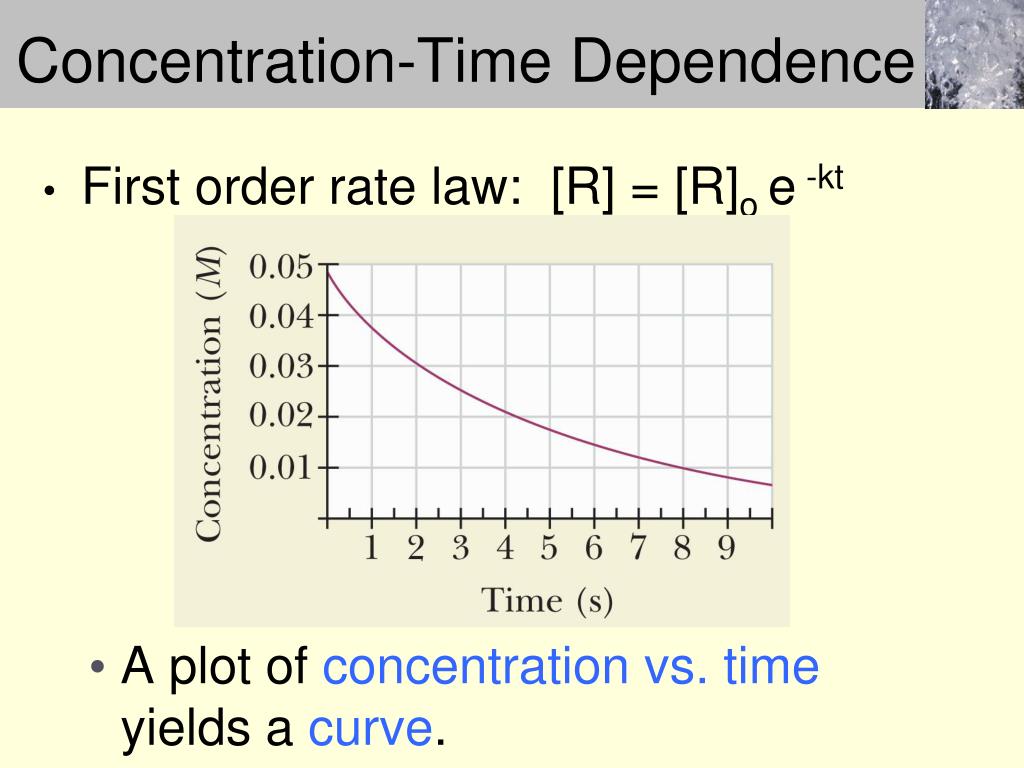
A Plot Of The Concentration Of The Reactant Versus Time Yields A Straight Line.
2021 Gmc Sierra 10-speed Transmission Problems, 2020 Silverado 1500 10 speed transmission issues, 7.87 MB, 05:44, 38,791, crkdtoes, 2021-01-25T22:55:06.000000Z, 19, New 2021 Cayenne Red Tintcoat GMC Sierra 1500 Crew Cab Short Box 4, www.taylorsautomaxbuickgmc.com, 960 x 540, jpeg, at4 denali buick yakima slt cayenne tintcoat peterson trim ritchey chateauguay daytona jerseyville elevation sle vin, 12, 2021-gmc-sierra-10-speed-transmission-problems, KAMPION
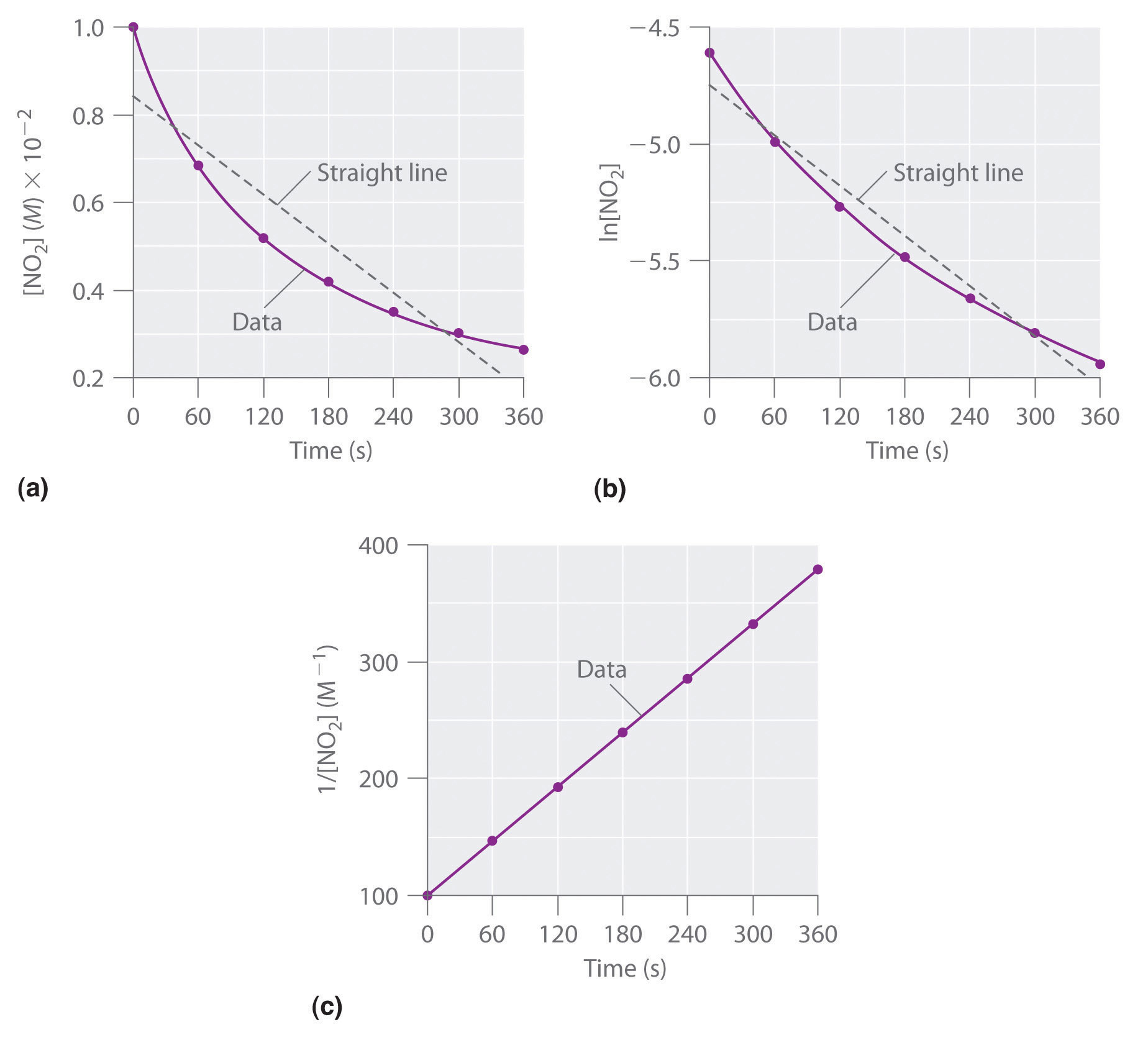
Indi cate the order of reaction consistent with each observation. A plot of the concentration of the reactant versus time yields a straight line. A plot of the inverse of the concentration versus time yields a straight. A plot of the concentration of the reactant versus time yields a straight line.
Which order of reaction would have a plot of the concentration versus time building a straight line. So first of all, it says concentration versus time. A plot of the natural log of the concentration of the reactant versus time yields a straight line. Show transcribed image text the data below were collected for this reaction: Ch3ci(g) + 3ci2(g) rightarrow cci4(g) + 3 hci(g) write an expression for the reaction. With [a] being the concentration of reactant a at time t, and k being the rate constant. [a]0 is then the initial concentration of a at time zero. T yielded a straight line, this reaction has a rate law of: R(t) = k[a] because we know m = 1 at this point.
14.7: Reaction Kinetics: A Summary - Chemistry LibreTexts

CHM 1046
PPT - Chapter 13 Chemical Kinetics PowerPoint Presentation, free
CHM 1046
Methods of Determining Reaction Order
12.1 Chemical Reaction Rates – Chemistry 112- Chapters 12-17 of
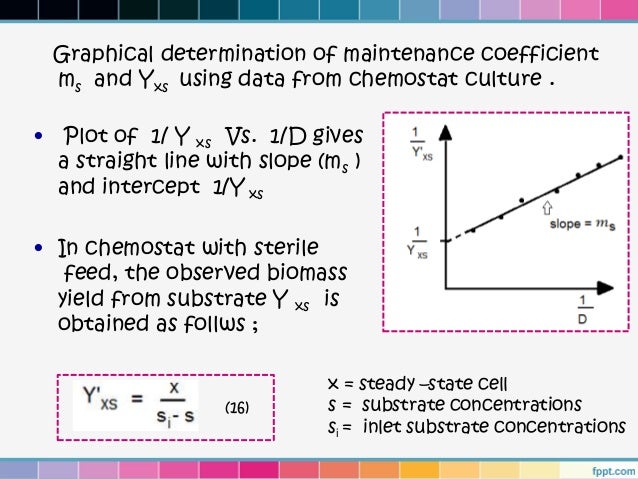
reactor engineering part 3
Zero-Order Kinetics (Constant Rate Processes) - Pharmacokinetics
PPT - Chapter 13 Chemical Kinetics PowerPoint Presentation, free

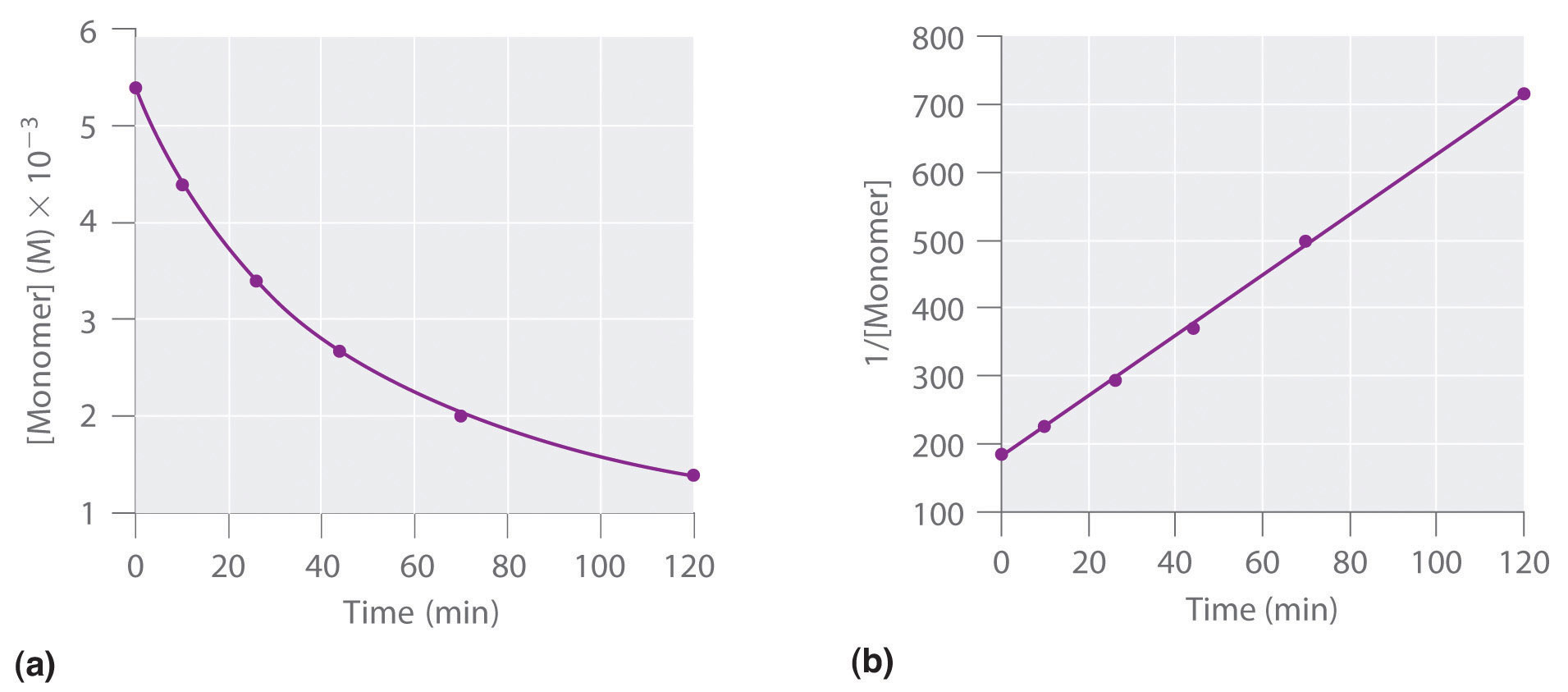
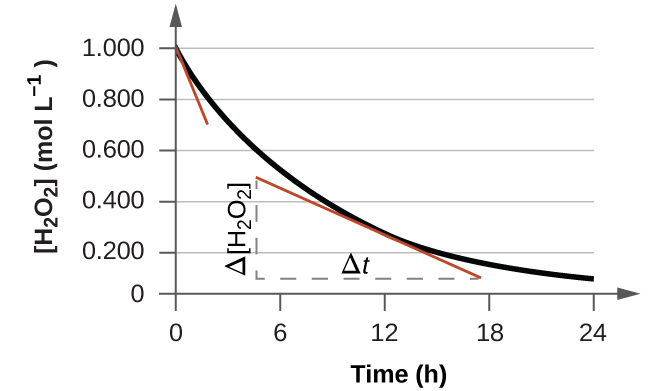
This reaction was monitored as a function of time…



Komentar
Posting Komentar